About AGAMREE®


Why AGAMREE?
AGAMREE is approved to treat Duchenne muscular dystrophy (DMD) in patients as young as 2 years of age
AGAMREE makes my legs less tired.
– Gaven, a real DMD patient taking AGAMREE

Download an educational brochure to help answer any questions you may have about AGAMREE
About AGAMREE
AGAMREE was developed to uncouple the anti-inflammatory effects of corticosteroids commonly used in DMD from certain side effects
While the way AGAMREE works to treat DMD is not fully understood, it is thought that AGAMREE treats DMD through its strong anti-inflammatory action
Effectiveness in Clinical Studies
AGAMREE is clinically proven to improve muscle strength and function in boys living with DMD
AGAMREE 6 mg/kg/day Improved Muscle Strength and Function
Boys treated with AGAMREE 6 mg/kg/day…

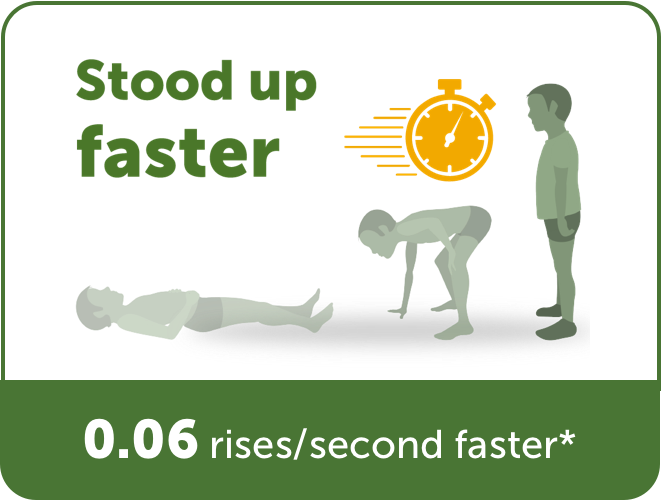
Time to Stand Test
(How fast a patient can stand from
lying on their back)


6-Minute Walk Test
(Distance a patient can walk in 6 minutes)


Time to Run/Walk 10 Meters
(How fast a patient can run or walk 10 meters)
*Compared to placebo (sugar pill).
Now that we’ve been on it for 6-ish months, his tummy problems are gone. He’s growing again. He’s happier overall, and he’ll tell you that he just feels better. He says his legs don’t hurt as much.
– Jessica, Gaven’s mom
Side Effects With AGAMREE in Clinical Studies
What are the most common side effects associated with AGAMREE?
In clinical studies in boys living with DMD, the most common side effects with AGAMREE treatment included facial puffiness or cushingoid features, psychiatric disorders, vomiting, weight gain, and vitamin D deficiency. These are not all the possible side effects of AGAMREE.
Call your healthcare provider for medical advice about side effects.
Includes the following adverse reactions that occurred more frequently in the AGAMREE group than in placebo: abnormal behavior, aggression, agitation, anxiety, irritability, mood altered, sleep disorder, and stereotypy.
*Includes the following adverse reactions that occurred more frequently in the AGAMREE group than in placebo: abnormal behavior, aggression, agitation, anxiety, irritability, mood altered, sleep disorder, and stereotypy.
Learn about AGAMREE dosing and administration
Catalyst Pathways® offers a range of free, personalized support and services
